Who should get tested for non-Aspergillus mold infections? What tests should they receive? Learn more about the tests for these molds.

DIAGNOSTIC TESTING
 Diagnosing a patient with non-Aspergillus mold infection can be challenging, even for experienced clinicians. There are a few reasons why diagnosis is so difficult.
Diagnosing a patient with non-Aspergillus mold infection can be challenging, even for experienced clinicians. There are a few reasons why diagnosis is so difficult.
- There are countless fungi that can cause non-Aspergillus molds infection. With increasing temperatures and more people on immunosuppressive drugs, the list of fungi that can cause human disease continues to grow. This means more fungi to tell apart.
- Growing fungi in a microbiology lab can take weeks, delaying identification of the specific fungi causing the infection.
- Other laboratory and imaging tests are not specific for non-Aspergillus mold infection.
- Other types of infections and medical conditions may mimic the signs, symptoms, and appearance on medical imaging of an infection with a non-Aspergillus mold.
 Because of the diagnostic challenges, healthcare providers often make a preliminary diagnosis based on the part of the body that is infected. Patients may even be started on antifungal treatment before the medical team reaches a final diagnosis and has identified the specific fungal pathogen. For example, an immunocompromised patient with fever, cough, and a computed tomography (CT) scan showing infection in their lungs may be diagnosed with a fungal pneumonia and started on antifungal treatment. The medical team may later identify the fungus as Rhizomucor pusillus, update their diagnosis to “pulmonary mucormycosis,” and adjust treatment as needed based on their final diagnosis.
Because of the diagnostic challenges, healthcare providers often make a preliminary diagnosis based on the part of the body that is infected. Patients may even be started on antifungal treatment before the medical team reaches a final diagnosis and has identified the specific fungal pathogen. For example, an immunocompromised patient with fever, cough, and a computed tomography (CT) scan showing infection in their lungs may be diagnosed with a fungal pneumonia and started on antifungal treatment. The medical team may later identify the fungus as Rhizomucor pusillus, update their diagnosis to “pulmonary mucormycosis,” and adjust treatment as needed based on their final diagnosis.
The Importance of the History of Present Illness
To start the diagnostic process, the medical team will interview the patient to collect relevant details about the patient, their medical history, current symptoms, and a timeline of recent activities (examples include travel history, animal exposure, new medications, etc.). Collectively, these details are called the “history of present illness.” Sometimes the history of present illness is documented in a patient’s chart as the “HPI.” You should give your doctor the information needed to make a diagnosis. Details from the HPI are critical to identifying a potential infection of non-Aspergillus mold. Some examples of key details:
- Prolonged and severe immune system dysfunction increases the overall risk to develop any kind of non-Aspergillus mold infection.
- Trauma or wounds that occurred during a severe weather event like a tornado. For example, following a 2011 tornado in Joplin, Missouri, there was a cluster of non-Aspergillus mold infections caused by Apophysomyces.
- Persistent fever and a new rash or skin lesions in a patient with neutropenia (an extremely low number of neutrophils, an important type of cells in our immune system) is highly concerning for infection with Fusarium spp.
- Pneumonia that develops after a near drowning has been associated with Scedosporium apiospermum.
- Patients with an infection that breaks through while on a preventative antifungal treatment (usually voriconazole or an echinocandin), is concerning for mucormycosis.
The medical team will order additional diagnostic testing informed by the details obtained in the HPI. There are three common types of testing used to help diagnose non-Aspergillus mold infections: 1) radiographic testing, 2) microbiology testing, and 3) histopathologic analysis.
Imaging
 X-ray, CT scan, and magnetic resonance imaging (MRI) are the most common types of radiographic studies used to help diagnose non-Aspergillus mold infections. The type of imaging and parts of the body imaged depends on the site of the presumed infection. For example, a CT scan of the chest would be useful for diagnosing a fungal pneumonia, but an MRI of the head and sinuses would be better to diagnose invasive fungal sinusitis. Findings in each type of imaging study can be variable based on the type of fungus, the status of the patient’s immune system, and the time a patient has been infected.
X-ray, CT scan, and magnetic resonance imaging (MRI) are the most common types of radiographic studies used to help diagnose non-Aspergillus mold infections. The type of imaging and parts of the body imaged depends on the site of the presumed infection. For example, a CT scan of the chest would be useful for diagnosing a fungal pneumonia, but an MRI of the head and sinuses would be better to diagnose invasive fungal sinusitis. Findings in each type of imaging study can be variable based on the type of fungus, the status of the patient’s immune system, and the time a patient has been infected.
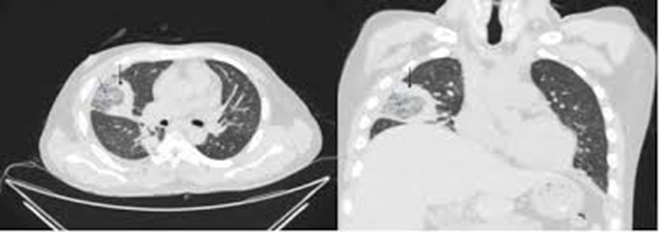
Because fungal pneumonia is the most common presentation of non-Aspergillus mold infection, the following is a list of common imaging findings on a chest CT.
- Halo sign: a lung nodule or mass surrounded by ground glass (hazy or fuzzy appearing) opacities suggesting hemorrhage from invasion of the fungus into blood vessels (angioinvasion)
- Reversed halo sign or Atoll sign: a ground glass opacity surrounded by a denser consolidation. A reversed halo sign is shown in Figure 1.
- Bird’s nest sign: a subtype of the reversed halo sign. Occurs when irregular lines are present within the central ground glass opacity mimicking the appearance of a bird’s nest.
- Air-crescent sign: a peripheral arc of air (appears black on CT scan) surrounding a lung lesion caused by fungal infection. This may be a good prognostic sign as it may represent improving immune system function.
- Vascular invasion: CT scans using intravenous contrast can detect abnormalities within blood vessels. Erosion into a blood vessel is a common finding in both fungal pneumonia as well as some types of cancer.
Figure 1. Close-up of a chest X-ray showing a reversed halo sign in a patient with mucormycosis. The arrows show the reversed halo sign in both views. Image from Reversed_halo_sign_in_pulmonary_Mucormycosis_-Dr_Piyush_Kumar.jpg (377 × 133 pixels, file size: 7 KB, MIME type: image/jpeg)
Microbiology Testing
This type of testing can be divided into two groups, culture-based and non-culture-based tests.
 Culture
Culture
Culture-based microbiology tests are aimed at isolating and growing the fungus causing a patient’s infection.
- Source: Blood, respiratory samples (like sputum or bronchoalveolar lavage [BAL]), cerebrospinal fluid, wound swabs, and tissues taken as a biopsy or during surgery of locations suspected to have non-Aspergillus mold infection
- What it’s testing for: The presence of live fungi capable of growing in culture
- When it is used: Cultures are obtained when non-Aspergillus mold infection is suspected in a particular location.
- Advantages: Establishes a definitive diagnosis and can help identify the specific fungal pathogen
- Disadvantages: If sampling a non-sterile site, isolation or growth of a fungus in culture may reflect contamination rather than true infection. It can take days to weeks for some non-Aspergillus molds to grow, delaying definitive diagnosis. Sometimes the mold may not grow at all, decreasing the sensitivity of this test.
Non-Culture-Based Testing
![]() Other diagnostic testing for non-Aspergillus mold infections do not require the mold to grow in culture. These tests offer more rapid testing results and can provide critical information while awaiting culture or histopathology results. However, unlike fungal culture, these tests do not provide a definitive diagnosis due to the high cross-reactivity between different fungi and each test.
Other diagnostic testing for non-Aspergillus mold infections do not require the mold to grow in culture. These tests offer more rapid testing results and can provide critical information while awaiting culture or histopathology results. However, unlike fungal culture, these tests do not provide a definitive diagnosis due to the high cross-reactivity between different fungi and each test.
Galactomannan (GM) antigen test (via enzyme immunoassay, GM-EIA)
- Source: Blood or BAL fluid
- What it’s testing for: GM is a polysaccharide (sugar) that makes up part of the cell wall in certain fungi. This test is intended to specifically test for Aspergillus GM, though other non-Aspergillus molds like Fusarium can cross-react with this test and produce positive results. A negative GM test result can also be helpful. For example, the fungi that cause mucormycosis do not produce a significant amount of GM and should be considered when a fungal infection is suspected and the GM test is negative. The test is performed using a process called EIA in the laboratory. In some cases, it is also tested with a lateral flow assay in a point-of-care test (like a COVID-19 test or a pregnancy test).
- Advantages: Galactomannan is easy to measure in serum. It provides a quantitative result, so it is useful for monitoring response to treatment over time.
- Disadvantages: The test is not very sensitive. False positives can occur in the presence of other non-Aspergillus molds like Fusarium and other fungi like Histoplasma. Additionally, exposure to certain substances can cause a false positive in rare circumstances.
Beta-D-Glucan
- Source: Blood or cerebrospinal fluid
- What it’s testing for: Beta-D-glucan is polysaccharide (sugar) found in the cell walls of certain fungi. This test is very non-specific and often simply reflects the presence of any fungi, not necessarily a specific fungal infection. A negative test can also be useful, as some types of fungi make very little Beta-D-glucan, like the fungi causing mucormycosis. Mucormycosis should be suspected when there is high concern for fungal infection and a negative Beta-D-glucan test.
- Advantages: Beta-D-glucan is easy to measure in serum and has a quick turnaround time. It provides a quantitative result, so it may be used for monitoring treatment response over time.
- Disadvantages: The test is very unreliable for establishing a diagnosis. The test is low specificity for any specific fungus. There are a lot of false positives with the test.
Polymerase Chain Reaction
- Source: Blood, and BAL fluid
- What it’s testing for: Fungal DNA
- Advantages: PCR is highly specific and can usually identify the specific fungal species causing an infection.
- Disadvantages: PCR testing is not completely standardized yet in the United States. There are only a few commercially available assays, so is not readily available in many centers.
Metagenomic Next-Generation Sequencing (NGS)
- Source: Blood, tissue samples (commonly from formalin-fixed paraffin-embedded tissue)
- What it’s testing for: Fungal DNA. Metagenomic HGS provides a readout of thousands of infectious agents in one test. It can test for most clinically relevant fungi using combinations of 18s, 28s, or internal transcribed spacers (ITS) primers.
- Advantages: The test is relatively non-invasive, quick, and specific. It can also identify co-infections.
- Disadvantages: Unless paired with histopathology and a compatible clinical syndrome, metagenomic NGS can be too sensitive and amplify any fungal DNA present (cause positive testing), even if the fungus is not causing infection. The test has not been extensively studied and validated against other fungal tests in large groups of patients.
Histopathology
 Source: Samples (usually biopsy or other tissue specimens) from the respiratory tract, sinuses, or other body regions where non-Aspergillus mold infection is suspected
Source: Samples (usually biopsy or other tissue specimens) from the respiratory tract, sinuses, or other body regions where non-Aspergillus mold infection is suspected- What it’s testing for: Evidence of non-Aspergillus mold invading tissue when the sample is looked at under a microscope. The various non-Aspergillus molds can sometimes be distinguished based on their appearance. For example: fungi from the group mucormycetes grow as broad hyphae without septations; this appearance is often described as ribbon-like hyphae, as shown in Figure 2.
- When it is used: Testing for invasive infection with non-Aspergillus molds in the particular body region (presence of hyphae) as well as evidence of damage to the tissue
- Advantages: Helps definitively establish invasive fungal infection
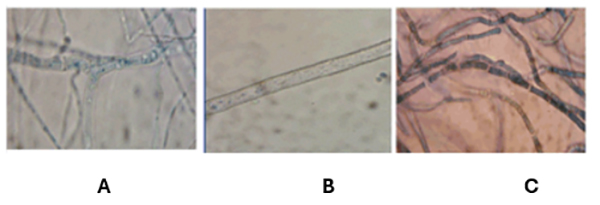
- Disadvantages: This test does not always detect all cases of invasive fungal infection regardless of specific pathogen. In addition, the appearance of fungi under the microscope can be confusing. For example, Fusarium can look very similar to other fungi like Aspergillus and form septate hyphae that also branch at acute angles, as shown in Figure 3. Therefore, it is important to interpret histopathology in conjunction with culture and other tests.
Figure 2. Image of Rhizopus oryzae from a patients with mucormycosis. This image, taken at a magnification of 600 times, shows flat, ribbon-like hyphae and an immature sporangium. Image reproduced from CDC/DR Lucille K. Georg.
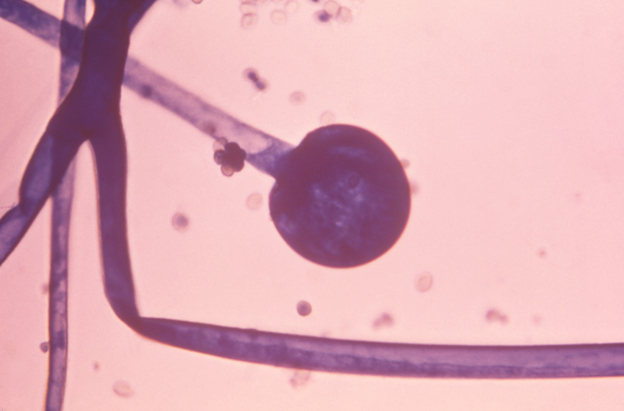
Figure 3. Comparison of the appearance of Aspergillus and Fusarium in histopathology. Both Aspergillus and Fusarium have hyphae that branch in acute angles (less than 90 degrees) with septa (divisions). Panels A and B show Aspergillus, while Panel C shows Fusarium. Note how similar these organisms look. Permission pending.
Frequently Asked Questions
This is a great point. Sometimes it is very hard to tell the two apart. Mold infections in the lung can look like a lung tumor, and molds can “spread” to the brain or other parts of the body like a cancer would. That’s where diagnostics become very important. Suspicious spots will most likely need to be biopsied to determine if the illness is caused by cancer or an infection caused by a mold and to establish the right treatment.
- Why do you suspect that I have a non-Aspergillus mold infection?
- What test will you order to check?
- How is the test performed? Is it a blood draw? Or something more invasive?
- Will you do any imaging?
- What does the test measure?
- How soon will the results get back?
- Might you require additional testing?
- What happens if the test is positive for a non-Aspergillus mold?
![]()

